DEMO TEST
Question 1 / 26
The following schematic diagram represents the fractional distillation of crude oil (crude petroleum) in industry. The letters A to E denote different products obtained from the distillation.
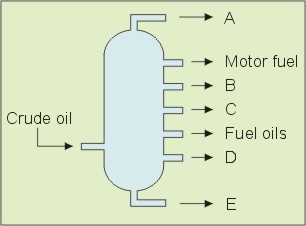
Question: Which one of the fractions A to E has the lowest boiling point range?
Answer:Question 2 / 26
Which one of the substances listed below is not an element ?
Question 3 / 26
What is the change in oxidation number (oxidation state, valency) of manganese in the reaction represented by the following equation:
MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O
Question 4 / 26
Which of the following substances are liquid at room temperature and standard pressure?
Question 5 / 26
Which of the following does not lead to an increase in the nitrogen content of soil?
Question 6 / 26
The process whereby hydrocarbons of high molecular mass are converted into hydrocarbons of low molecular mass is called:
Question 7 / 26
The ability to conduct electricity readily is a property of ...
Question 8 / 26
How is the presence of ions in an aqueous solution most directly detected?
Question 9 / 26
The main component of paper is ...
Question 10 / 26
A sample of an aqueous sodium chloride solution containing 8 g of sodium chloride in 100 ml of solution is concentrated to half of its original volume.How many grams of sodium chloride are contained in 25 ml of the new solution?
Answer:
Answer:
Question 11 / 26
The relative atomic mass of carbon (C) is about 12. Approximately, how many atoms are contained in 6 g of carbon?
Question 12 / 26
What is the minimum weight of sodium chloride (NaCl) that is needed to prepare 7.10 g of chlorine ?Relative atomic masses: Na = 23.0 ; Cl = 35.5
Question 13 / 26
The graph below shows the solubilities (in g solute/100 ml water) of sodium nitrate and sodium chloride at different temperatures. Refer to it to answer this question.100 g of sodium nitrate and 100 g of sodium chloride are added to 100 cm3 of water at 60°C. The mixture is stirred until no more solid dissolves. It is then filtered, keeping the temperature at 60°C.
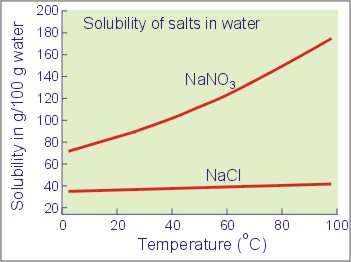
Question 14 / 26
From the following, select the three bases that are most commonly used in the chemical industry.
Question 15 / 26
Apart from carbon, proteins always contain:
Question 16 / 26
According to the chemical reactivity series, metals can displace other metals from their solutions.Which of the following metals will displace copper from its solution?
Gold - Iron - Magnesium - Silver - Zinc
Question 17 / 26
An aqueous solution of sodium iodide is electrolysed with platinum electrodes.What is the main product at the cathode (negative electrode) ?
Question 18 / 26
Correct statements about the material known as PVC include:
Question 19 / 26
The two non-metals in the following group are:
Question 20 / 26
When marble pieces are added to hydrochloric acid, the following reaction takes place:
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)Which of the following actions would increase the rate of formation of carbon dioxide?
Question 21 / 26
Compounds that have high melting points, conduct electricity in the molten phase and are often soluble in water are
Question 22 / 26
From the list, select the gases that can be collected using the apparatus shown in the diagram on the left.
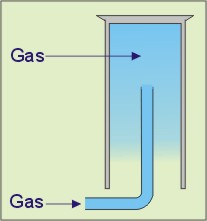
Question 23 / 26
Oxides which produce alkaline solutions when mixed with water include:
Question 24 / 26
Calculate how many moles of ethanoic acid (CH3COOH: relative molecular mass 60) may be produced from 92 g of ethanol (CH3CH2OH: relative molecular mass 46). Answer:
Question 25 / 26
The reaction between zinc (Zn) and hydrochloric acid produces...
Question 26 / 26
Which of the following would yield the greatest amount of useful energy ?